Holder and "exploitant" of several Marketing Authorisations (MAs). Intsel Chimos increases the value of the medecines developed by international pharmaceutical companies wishing to access the French market
Our strong market knowledge and experience makes Intsel Chimos a valued partner in the areas of operations, distribution to both hospitals and retail pharmacies, regulatory affairs service, and quality assurance management.
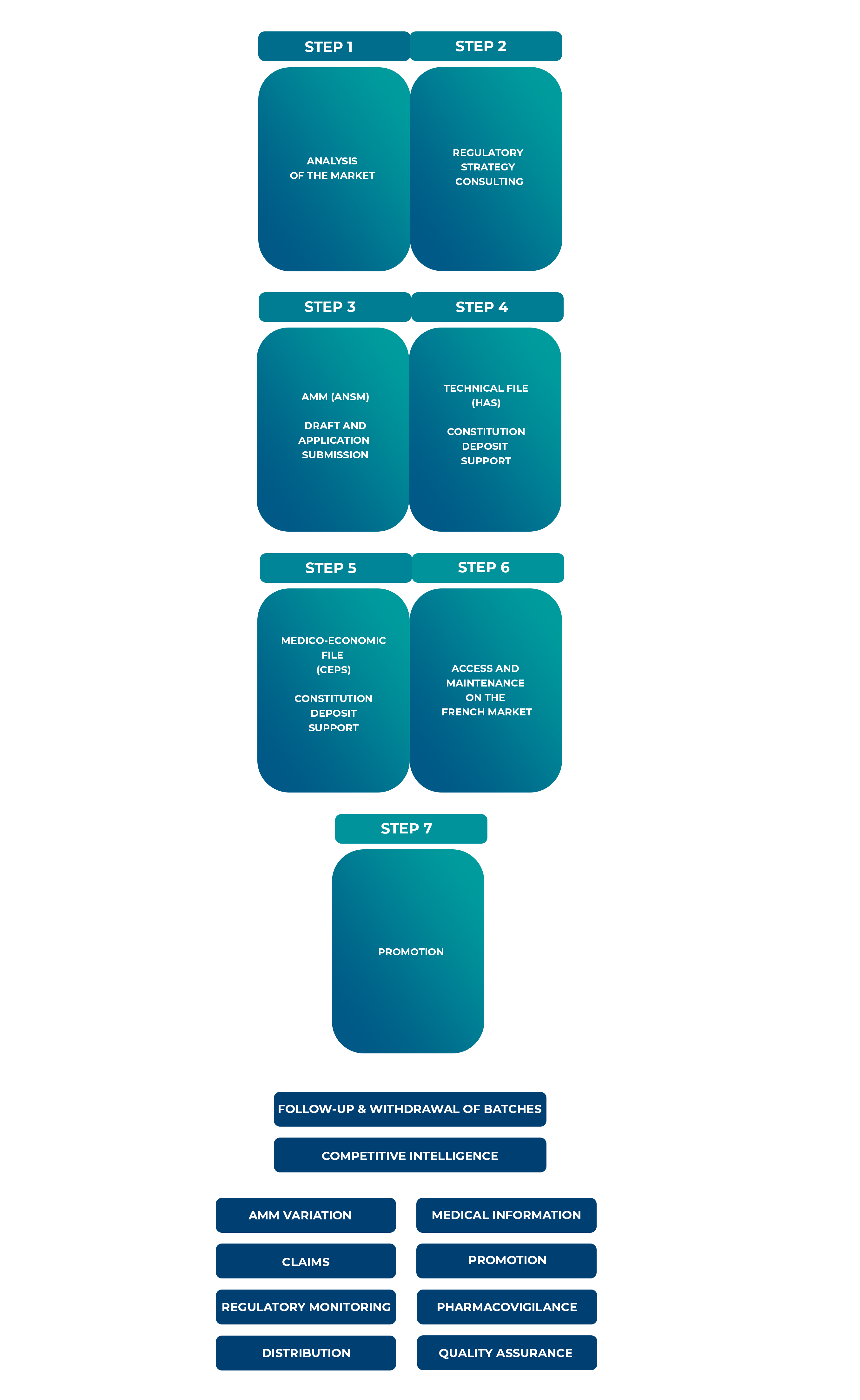
Our expertise in the French and the European healthcare markets allows us to advise and support our partners in the development of the most appropriate regulatory strategy for their products and to assist them throughout the necessary marketing steps.
Intsel Chimos pays particular attention to conducting its collaborations in complete transparency and is committed to investing throughout the life of the medicines (distribution, batch monitoring and recall, medical information, pharmacovigilance, MA variations. promotional information. etc.).
Intsel Chimos, in accordance with the information Charter, is licensed to canvass or prospect to promote medicines. All these actions are carried out in compliance with our Quality Policy which guarantees the management of the products under ideal conditions.

lntsel Chimos , as the "exploitant" laboratory, supports biotech and pharmaceutical companies in providing access to their products, whether they are under development or at the early access phase.

The early access program (‘AAP’) is submitted on behalf of the pharmaceutical companies holding the MA rights in order to facilitate patients’ access to these treatments. (that sometimes do not have yet their MA. )
These medicines are intended to be marketed. As such, they need to comply with a protocol for therapeutic use and the monitoring of patients (‘TUP’), which requires the involvement of a French “exploitant” laboratory.
In order to comply with the regulatory framework and to guarantee the quality of the medicines with the regulatory authorities, Intsel Chimos adheres to a strict quality policy and control.




lntsel Chimos partners with your organization across all stages.