Upon request of a Health Care Professional (HCP) and with the approval from ANSM, lntsel Chimos provides compassionate access to drugs that do not have a marketing authorization (MA) in France to allow HCP to treat rare or serious pathologies that have reached a therapeutic impasse.

As part of its commitment to early access to innovation, lntsel Chimos is authorized to distribute products intended for human experimentation. This delicate operation requires specific expertise and is deeply embedded in all stages of our supply chain.
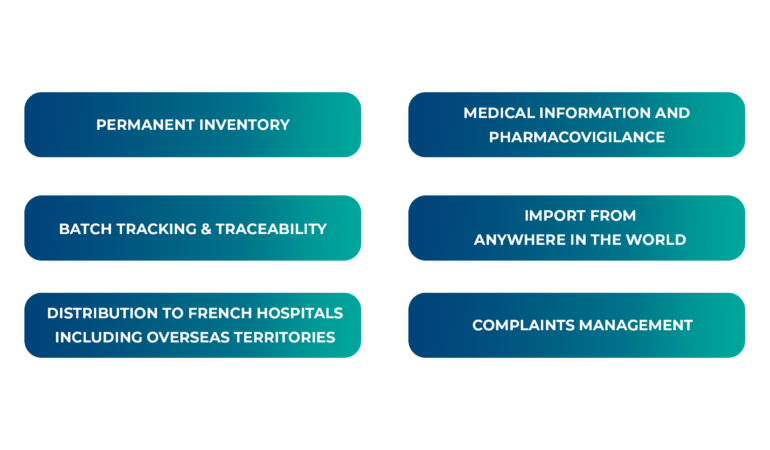
Intsel Chimos team takes every precaution to handle and manage these sensitive products within their own strict regulatory framework.
We are committed to supporting clinical studies sponsors throughout the duration of their trials, in particular by guaranteeing the quality of the handling, storage, safety and traceability of their products.
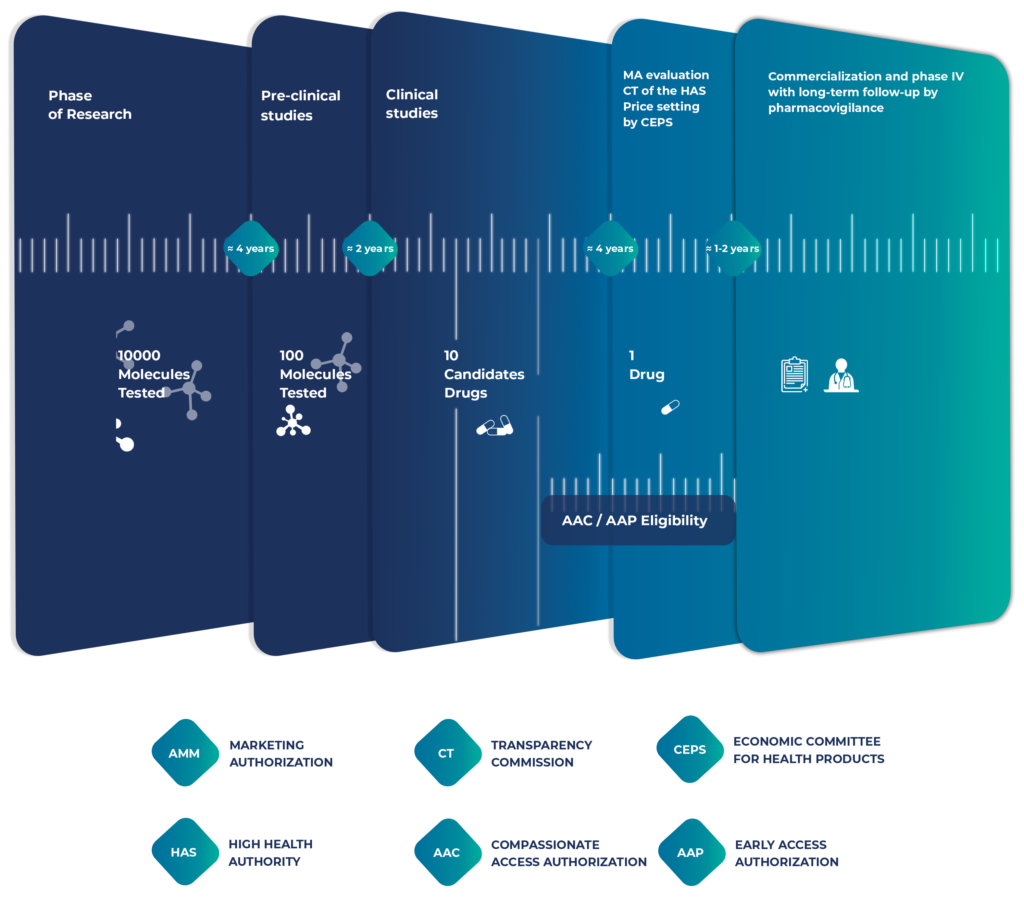
Based on our involvement in treating patients, our knowledge of AAC, and our experience on the French market, we are the partner of choice for pharmaceutical companies in their market access process, depending on the development phase of their pharmaceutical products.