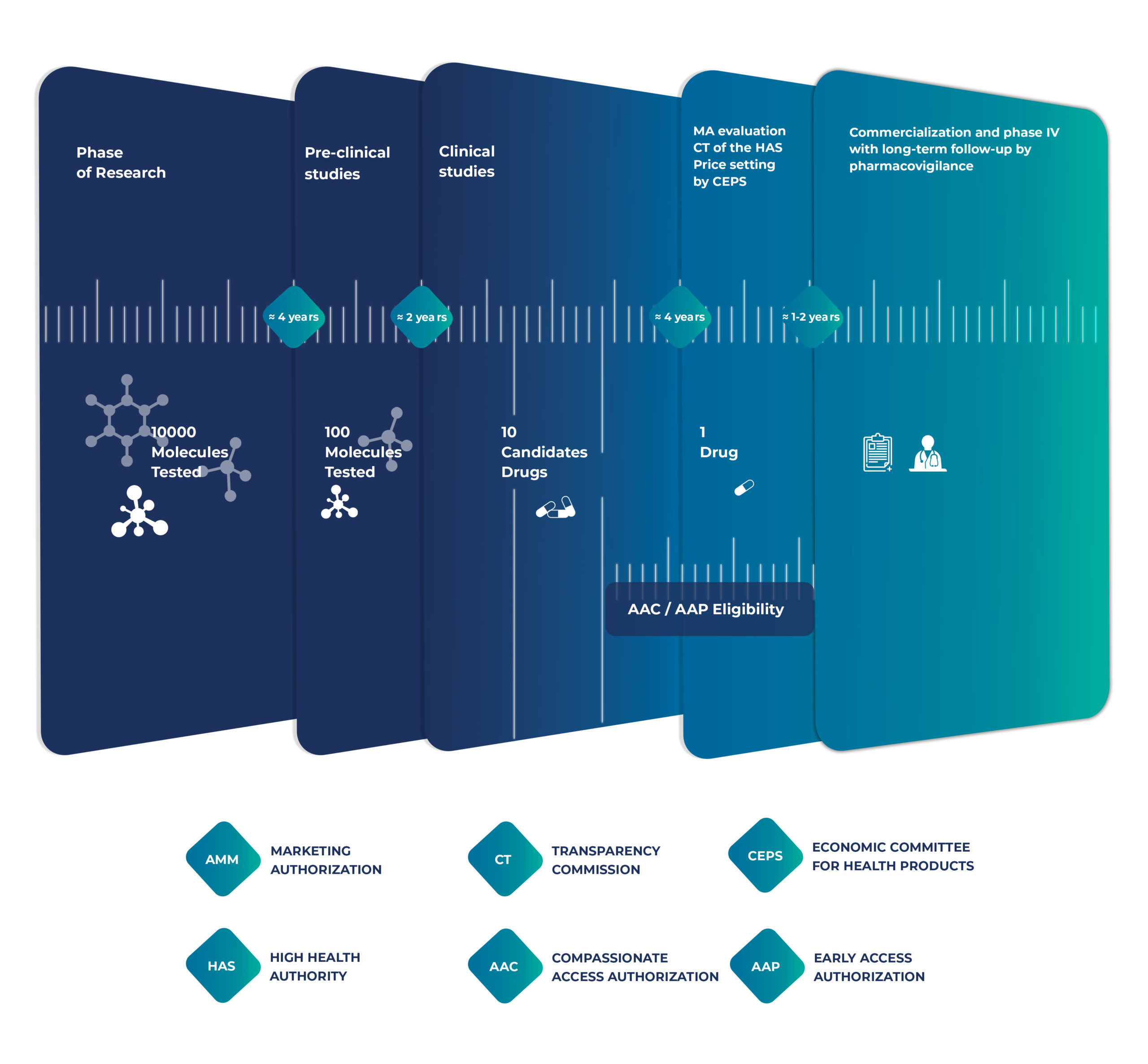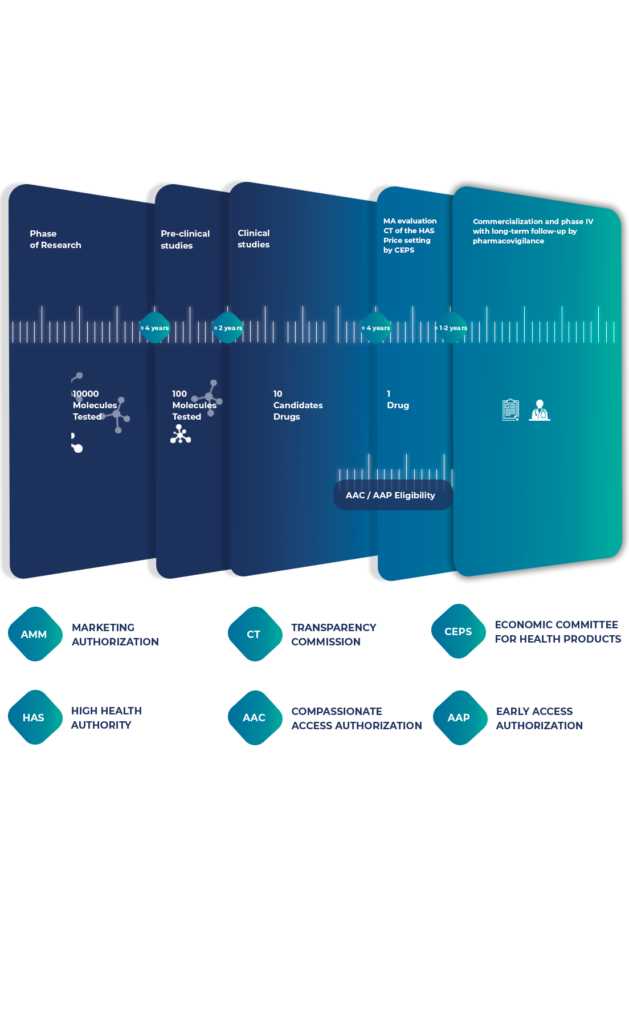
Intsel Chimos specializes in facilitating early access, providing comprehensive support to pharmaceutical companies introducing therapies into the French healthcare system — before and also after Marketing Authorization.
Compassionate Use (Autorisation Accès Compassionnel – AAC)
For patients suffering from serious or life-threatening diseases with no therapeutic alternatives, compassionate use offers a vital channel to access promising treatments still under development. These programs are granted on a case-by-case patient basis outside of clinical trials.
Our Role in Compassionate Use:
Early Access Programs (EAPs)
The Autorisation d’Accès Précoce (AAP) provides early access to innovative medicines for patients with serious conditions and rare diseases.
Our Role in Early Access:

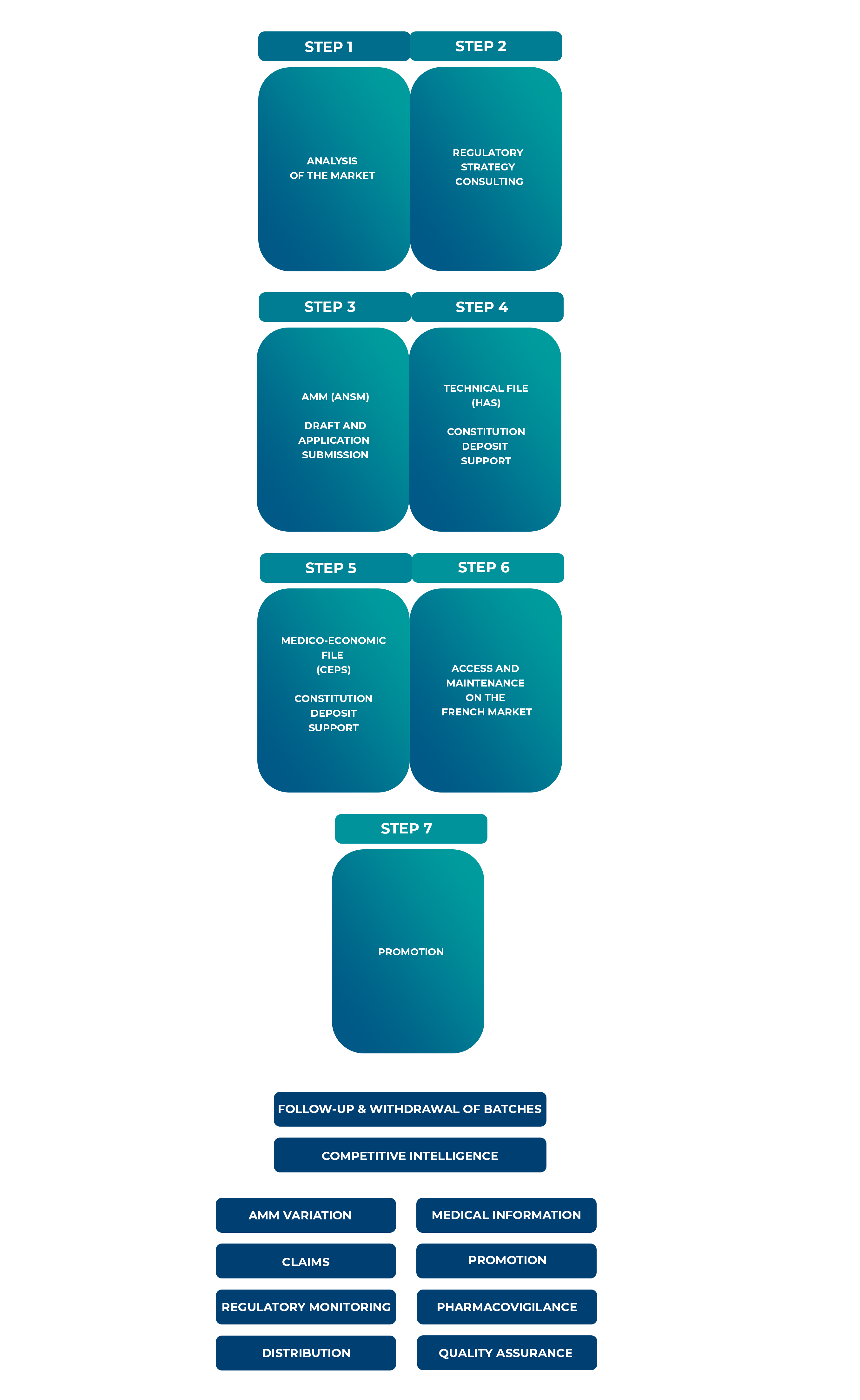
A strategic approach and market analysis is essential to ensure successful pharmaceutical market entry. It needs to be put in place well in advance of the launch of the product (be it under EAP or for products with a marketing authorization). This includes competition monitoring, price negotiations, and health technology assessments (HTAs). Also, our seamless transition services ensure uninterrupted availability of treatments from early access to full marketing.
Our Role in Market Access:
Intsel Chimos partners with your organisations across all stages.
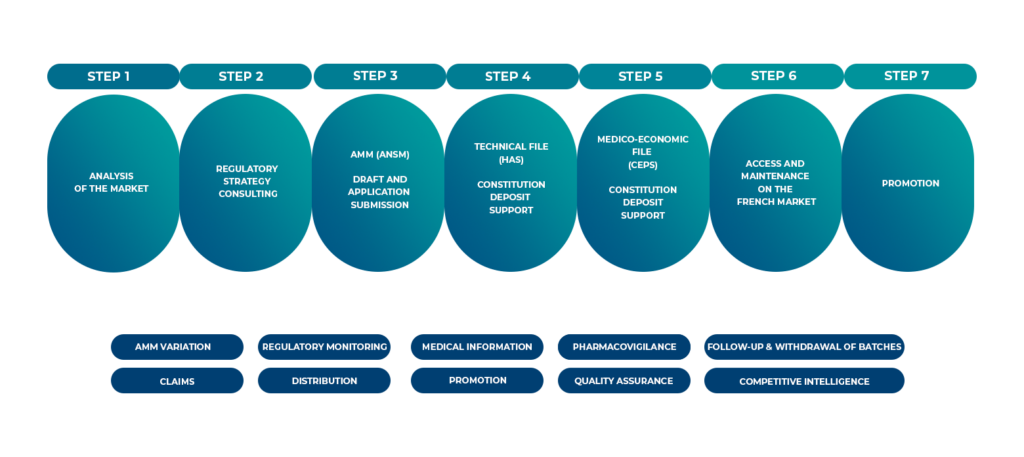

At Intsel Chimos, we facilitate the execution of clinical trials, ensuring precise data collection and management, to validate the efficacy and safety of new treatments in real life. Our services are adapted to meet the specific requirements of each study and are fully compliant with the GDPR regulation.
Data Management
Accurate data collection and management are critical to maintaining the integrity of a clinical trial and/or the efficacy and safety of a treatment, within the framework of a managed access program. We utilize advanced electronic data capture (EDC platform) systems to ensure real-time data entry, validation, and monitoring. Our data management practices align with regulatory standards, ensuring accuracy, completeness, and verifiability.
Clinical Trial Distribution
We provide comprehensive distribution services within clinical trials, ensuring timely and compliant delivery of investigational products.